Nursing & Healthcare News
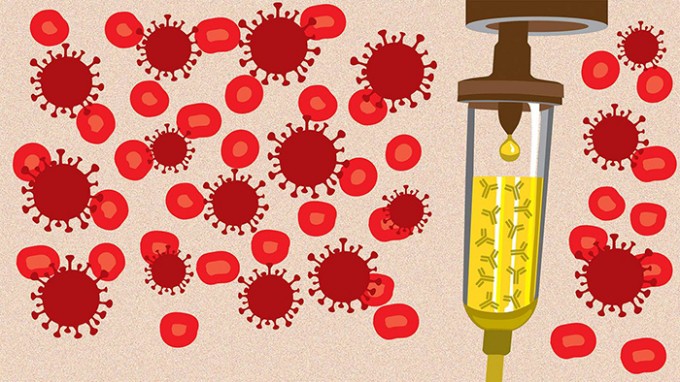
Convalescent Plasma Therapy
Seeking donations from recovered COVID-19 patients
- One donation could help up to four patients
- More than 2,300 participating patients at 1,663 clinical sites
- Therapy still experimental and only given to sickest patients
Do you have patients who have fully recovered from COVID-19? Ask them if they would consider donating plasma for an experimental treatment protocol.
Antibody-Rich Plasma
There’s still no approved treatment for COVID-19, but the FDA recently approved expanded access to an experimental treatment using convalescent plasma: blood plasma from patients who have recently recovered from the 2019 novel coronavirus. The theory is that the antibodies in the plasma could help the immune systems of other patients to better fight the disease.












