Nursing & Healthcare News
FDA Approves OTC Narcan
Over-the-counter naloxone will expand access to opioid overdose treatment
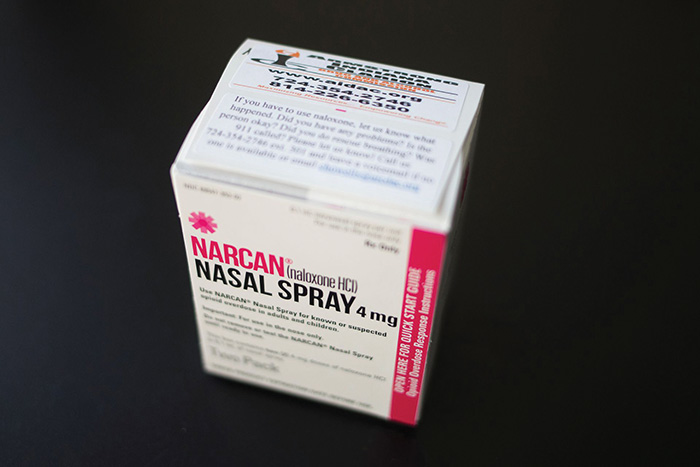
Some good news in the ongoing opioid crisis: The FDA recently gave the green light to over-the-counter (OTC) sales of Narcan nasal spray for opioid overdose reversal — the first such treatment available without a prescription in the U.S.
Saving Lives
If you’ve worked in the ED or in substance abuse treatment, you may have firsthand experience with Narcan or other overdose treatments with the same active ingredient: the opioid reversal agent naloxone.
By temporarily blocking the effects of opioids on the brain, naloxone can restore an overdose victim’s breathing within two to three minutes, greatly increasing their chances of survival. According to National Institute on Drug Abuse Director Nora Volkow, M.D., the biggest problem with this lifesaving drug has been making it accessible.



